Low Cell Optimization Module Overview
When performing ChIP-Seq on limited samples, maximizing retainment of the already precious sample is key. Active Motif's Low Cell Optimization Module enables Low Cell ChIP Kit users to monitor sample retainment as they progress through the protocol. This first-of-its-kind module includes "positive control" samples and antibody, as well as reagents to examine chromatin quantity and quality following sonication and ChIP enrichment. Using this module, researchers can monitor low cell samples throughout the ChIP protocol, identify and optimize steps responsible for sample loss, and save time and money by only moving forward with samples that will generate valuable ChIP-Seq data.
Low Cell Optimization Module Highlights
- "Positive control" samples and antibody enable users to optimize protocol steps without wasting precious samples
- Chromatin quantity and quality can be examined throughout the protocol, to quickly identify underperforming steps
- Sold separately from the Low Cell ChIP Kit, to facilitate optimization only when necessary
ChIP-Seq Data from Positive Control Jurkat cells
Examine Low Input Chromatin Throughout the ChIP Protocol
This module is intended to be used together with the Low Cell ChIP Kit, either to optimize protocol steps prior to using your precious samples, or to troubleshoot the point in the protocol responsible for sample loss. The Low Cell ChIP Kit is robust enough to work with both high abundance histone modifications and low abundance transcription factor proteins.
Don't Waste Your Precious Samples on Workflow Optimization
Positive control samples (Jurkat cell pellets) and antibody (AbFlex® H3K9ac) are included in the module for user optimization of the Low Cell ChIP protocol. This provides users the ability to optimize workflow conditions prior to using their low input, precious samples. These positive control reagents, not provided with any other low cell ChIP kit on the market, save users the frustration of optimizing and troubleshooting protocols with samples they care about.
Low Cell Optimization Module Data
Examine Low Input Samples Throughout the ChIP Protocol
With Low Cell ChIP, researchers can reduce the number of cells they are studying to improve their understanding of the complexities of protein-DNA interactions to a more limited population of cells. The assay is ideal if working with limited sample amounts, hard to culture cell lines, or small tissue biopsies. It is important to maximize retainment of limited samples throughout the ChIP protocol, which is why Active Motif has developed a module to examine samples at intermediate steps in the workflow: following chromatin preparation and ChIP enrichment (see purple steps on Days 1 and 4 below).
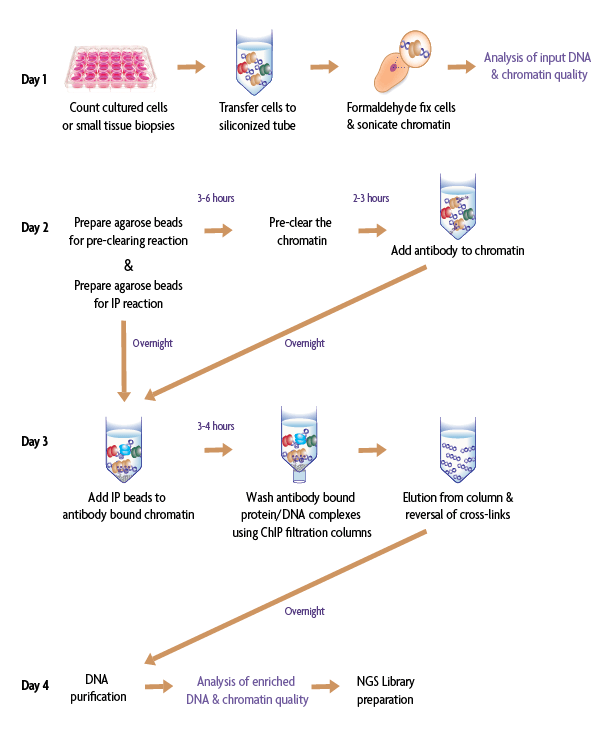
Figure 1: The Low Cell Optimization Module allows for optimization and troubleshooting when using the Low Cell ChIP Kit (Cat. No. 53086). This module contains a standardized sample and antibody, as well as reagents to examine input DNA and chromatin quality (Day 1) and enriched DNA and chromatin quality (Day 4).
Determine Sample Quantity Following Chromatin Preparation
Many limited samples used in the Low Cell ChIP Kit will be below the limit of detection for instruments commonly used for DNA quantification (e.g. Qubit). Therefore, we recommend a qPCR-based approach to examine samples following chromatin preparation, to ensure that you have retained enough sample to proceed through the protocol.
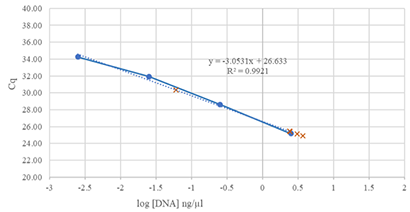
Figure 2: Using the Low Cell Optimization Module, a qPCR-based quantification of samples can be performed following chromatin preparation. DNA standards and GAPDH-2 primers were used to analyze DNA from Fixed Jurkat Cell Pellet samples (equivalent to 100,000 Jurkat cells).
Analyze Sample Fragmentation Following Chromatin Preparation
Accurate sample fragmentation is integral for high quality ChIP-Seq data. The Low Cell Optimization Module allows sample fragmentation profiles to be examined following chromatin preparation (Day 1), rather than following NGS library preparation (Day 4). Early examination of fragmentation allows users to optimize sample sonication efficiently, without wasting time and reagents on enrichment and library preparation.
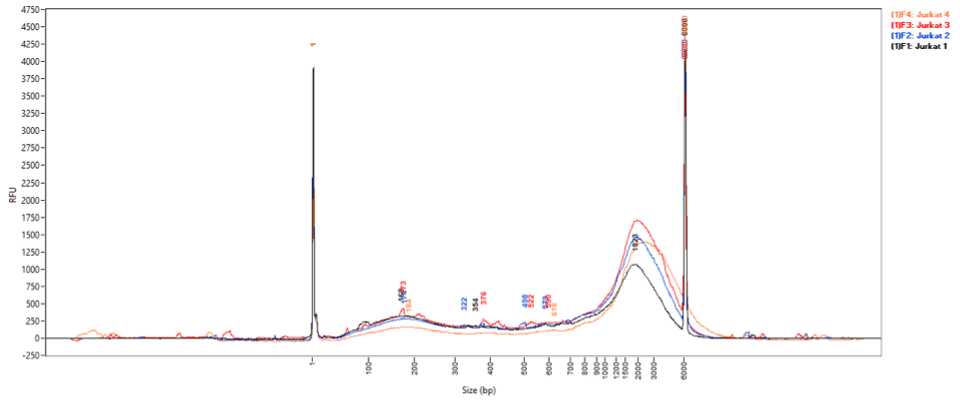
Figure 3: Using the Low Cell Optimization Module and a Fragment Analyser, profiles of solubilized chromatin were analyzed. High molecular weight peaks are commonly seen at this point in the protocol, but do not affect the ChIP enrichment or sequencing data.
Confirm Sample Enrichment Following ChIP
To ensure specific and successful antibody enrichment of your sample, we recommend using primers targeted towards positive and negative control regions to amplify and characterize fold-enrichment. The primers included in the Low Cell Optimization Module are intended for the H3K9ac antibody. If using another antibody, find primers appropriate for your target by examining Active Motif's ChIP Control qPCR Primer Sets.
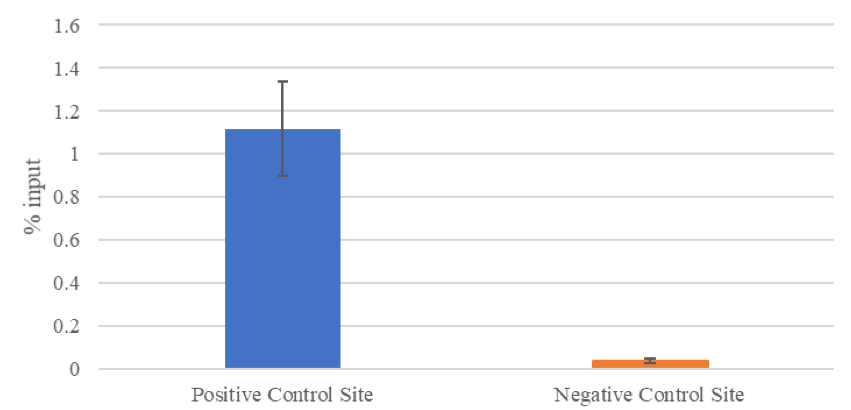
Figure 4: Using the Low Cell Optimization Module and the positive and negative control site primers, enriched chromatin from Fixed Jurkat Cell Pellet samples was analyzed. ACTB-2 and Hum Neg primers are specific to H3K9ac enrichment. These primers and antibody are all included in the Low Cell Optimization Module, along with positive control Jurkat cell pellets. Data shown here is an aggregate of 30 samples; average fold enrichment = 28-fold; p=5.66x10-5
Visualize ChIP-Seq Peaks from Positive Control Samples
Positive control reagents (Jurkat cell pellets, H3K9ac antibody, and control primer sets) are included in the Low Cell Optimization Module to allow users to become familiar with and troubleshoot the Low Cell ChIP Kit prior to using any of their precious samples. Following examination of chromatin quality and quantity, positive control samples can be sequenced to confirm that ChIP-Seq peaks appear as expected.
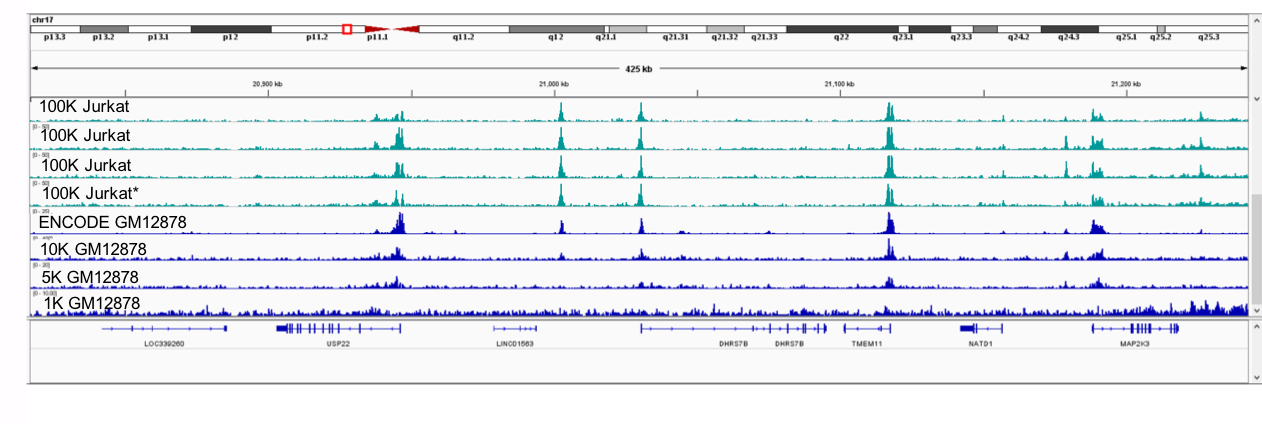
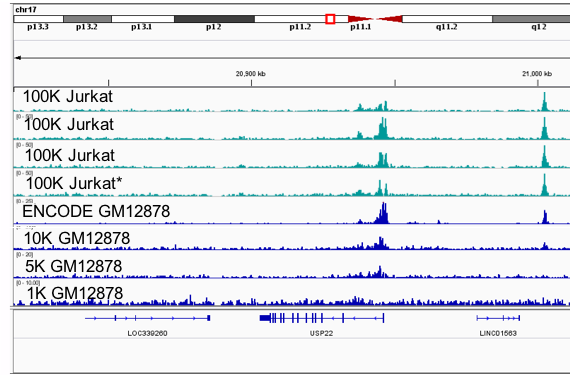
Figure 5: The Low Cell ChIP Kit and Low Cell Optimization Module were used to analyze enriched chromatin from (100,000 cell) Fixed Jurkat Cell Pellet samples (top 4 tracks, green). A section of chromosome 17 is shown here. The bottom Jurkat track (marked with *) exhibited much lower fold-enrichment of positive control regions, as analyzed by qPCR. As a result, slightly degraded peaks can be observed in this sample. For comparison, H3K9ac enrichment of GM12878 cells is also shown (blue). The top track is publicly available ENCODE data from 20 million cells, the lower tracks demonstrate peak degradation when using just 10,000, 5,000, or 1,000 cells.
Low Cell Optimization Module Contents
Please note that the Low Cell Optimization Module is shipped on dry ice and contains reagents with multiple storage temperatures inside. All components can be stored at -20°C prior to first use, then we recommend storing each component at the temperatures indicated below. All reagents are guaranteed stable for 6 months from date of receipt when stored properly. Each module includes the following components:
- Fixed Jurkat Cell Pellet (6 tubes); Store at -80°C
- RNase A; Store at -20°C
- DNA Standard AM1; Store at -20°C
- DNA Standard AM2; Store at -20°C
- DNA Standard AM3; Store at -20°C
- Human Pos Control Primer Set, GAPDH-2; Store at -20°C
- Human Neg Control Primer Set 1; Store at -20°C
- Carrier; Store at -20°C
- Human Positive Control Primer Set, ACTB-2; Store at -20°C
- AbFlex® Histone H3K9ac Antibody; Store at -20°C
Low Cell Optimization Module Documents
You might also be interested in:
| Name | Format | Cat No. | Price | |
|---|---|---|---|---|
| Low Cell Optimization Module | 6 rxns | 53085 | $435 | Buy |

