<< Back to MOTIFvations Blog Home Page
DNA Modifications Reveal the Splendor of the Plant Epigenetic Landscape

July 22, 2022
Table of Contents:
Introduction
An ever-increasing range of RNA and DNA modifications form a critical part of the epigenetic landscape of plants, with each contributing their specific distribution pattern and function. The ongoing discovery of novel modifications continues to add new color and shape to this garden of epigenetic delights, and recently, a band of green-fingered researchers published an exciting study that explored the presence and function of a novel N4-acetyldeoxycytosine (4acC) DNA modification in the genome of the model plant Arabidopsis thaliana. And if this fascinating study piques your interest, we also report on a handy protocol that makes detecting DNA modifications in plants a more straightforward task!
N4-acetyldeoxycytosine – A New DNA Modification Sprouts Up in Model Plant
A team from Nanjing Agricultural University (Jiangsu, China) headed by Yufeng Wu knew that nucleic acid modifications tended to come in pairs – given the appearance of a modification in RNA (e.g., 5-methylcytosine [m5C], 5-hydroxymethylcytosine [hm5C], and N6-methyladenosine [m6A]), an analog in genomic DNA usually “sprouts up” alongside (e.g., 5mC, 5hmC, and 6mA, respectively).
Multiple previous studies have described the presence of the N4-acetylcytosine/ac4C modification in RNA; Arango et al. linked increased ac4C levels in the human transcriptome to messenger RNA stability and translation efficiency, Tardu et al. employed advanced characterization techniques to describe the ac4C modification of yeast messenger RNA, and Sas-Chen et al. confirmed that ribosomal and transfer RNA also carried ac4C. As evidence for the N4-acetyldeoxycytosine/4acC analog in genomic DNA remained scant, Wang, Xie, Mao, and colleagues set out to evaluate the possible existence of this novel DNA modification in the model plant Arabidopsis thaliana. They recently described their exciting findings in Genome Biology.
After initially confirming the general abundance of 4acC in Arabidopsis by immunoblot after RNA depletion (with an antibody that recognizes ac4C in mRNA) and mass spectrometry-based analysis (UPLC-electrospray ionization-mass spectrometry (UPLC-ESI-MS/MS), the authors profiled the genome-wide distribution of 4acC by immunoprecipitation followed by sequencing (4acC-IP-seq). This sensitive technique revealed the presence of 4acC peaks distributed throughout euchromatin, the section of the genome that generally displays a more transcriptionally permissive nature.
A detailed analysis of this data revealed the presence of 4acC at around half of all expressed protein-coding genes in Arabidopsis, with 4acC generally encountered around transcription start sites and high levels correlating robustly with active transcription. Interestingly, the categorization of Gene Ontology (GO) terms associated with expressed genes associated with 4acC compared to those without suggested that 4acC-modified expressed genes probably participate in more diverse biological functions than expressed genes lacking 4acC.
An analysis of the potential interplay of 4acC with other modifications revealed that 4acC and 5mC occupied distinct genomic regions, with changes to 5mC levels failing to prompt a direct effect on the distribution of 4acC; however, genes modified with 4acC displayed higher 5mC levels in the CG context within gene bodies and lower 5mC methylation levels in CHG/CHH contexts (where H is A, C, or T).
Regions associated with 4acC also tended to co-exist alongside transcription-associated permissive histone modifications such as H3K4 di- and tri-methylation (H3K4me2/H3K4me3), H3K36 tri-methylation (H3K36me3), H3K9 acetylation (H3K9ac), and H3K14 acetylation (H3K14ac). While these histone modifications played a more dominant role in modifying gene expression than 4acC alone, 4acC-modified genes displayed higher expression levels than non-4acC-modified genes when they lacked permissive histone modification marks or possessed repressive histone modifications. While this suggested a positive role for 4acC in the absence of active histone modifications, 15% of 4acC peaks also displayed an overlap with the repressive H3K27 trimethylation (H3K27me3) modification, a level significantly higher than the percentage of overlap that would occur by chance. These findings suggest that 4acC, 5mC, and histone modifications cooperatively regulate gene expression in a complex manner.
This study also discovered a significant overlap of 4acC with DNase I hypersensitive sites (suggesting that 4acC-modified regions contain regulatory DNA elements) and binding sites for a wide range of transcription factors. The authors hypothesize that the presence of the 4acC facilitates transcription factor binding to support the tight regulation of gene expression.
Finally, the authors advanced their research beyond the model plant Arabidopsis and provided evidence for 4acC in the genomic DNA of rice and maize (whose genome displays greater complex, with 85% constituted by transposons) through mass spectrometry-based analyses. Importantly, the team also noted the presence of 4acC in the genomic DNA of mice and humans, thereby providing evidence that 4acC distribution and function may remain similar across varied species; however, the authors underscore the need to carry out 4acC-IP-seq in mammals to validate these findings and shed further light on the evolutional conservation/divergence of 4acC´s biological function.
While these findings reveal much about the distribution and role of the 4acC modification (a hitherto unknown DNA modification that has sprouted up in higher eukaryotes!), the authors note the need to continue this research to identify 4acC writers (acetyltransferases), erasers (deacetylases), and readers (4acC binding proteins) and further explore the complex role of 4acC in gene expression regulation and any crosstalk with other epigenetic modifications.
After N4-acetyldeoxycytosine, how many more DNA modifications will sprout up in model plants and contribute to the splendor of the plant epigenetic landscape?
Want to Detect Modified Cytosines in Plants? There´s a Protocol for That!
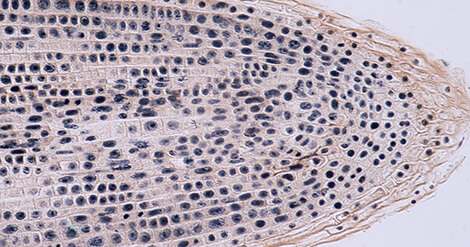
If this walk on the wild side of DNA modifications has put you in the mood to advance your epigenetic research, Marcos Viejo, Igor A. Yakovlev, and Carl Gunnar Fossdal from the Norwegian Institute of Bioeconomy Research (Ås, Norway) have sketched out a standardized protocol that will assist epigenetics researchers in detecting a variety of cytosine modifications in plant tissues.
This talented trio offers a detailed step-by-step protocol for the immunolocalization of modified species of cytosine in plant tissues and a handy troubleshooting section that should help to overcome most common issues. The sensitivity of their method, which employs enzyme-conjugated secondary antibodies, supports the detection of extremely low levels of oxidized 5mC forms in bud sections from the gymnosperm Norway spruce. Their findings included the detection of the highly abundant 5mC modification but also the presence of very low levels of 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC) and their heterogeneous distribution within the nuclei.
The details of this method incorporate each step of fixation and sectioning of dormant buds, followed by the immunolocalization of 5mC and its oxidized forms (permeabilization, antigen retrieval, immunodetection, and counteract staining and mounting) and imaging. What more do you need to get started!?
About the author

Stuart P. Atkinson, Ph.D.
Stuart was born and grew up in the idyllic town of Lanark (Scotland). He later studied biochemistry at the University of Strathclyde in Glasgow (Scotland) before gaining his Ph.D. in medical oncology; his thesis described the epigenetic regulation of the telomerase gene promoters in cancer cells. Following Post-doctoral stays in Newcastle (England) and Valencia (Spain) where his varied research aims included the exploration of epigenetics in embryonic and induced pluripotent stem cells, Stuart moved into project management and scientific writing/editing where his current interests include polymer chemistry, cancer research, regenerative medicine, and epigenetics. While not glued to his laptop, Stuart enjoys exploring the Spanish mountains and coastlines (and everywhere in between) and the food and drink that it provides!
Contact Stuart on Twitter with any questions
Related Articles
3’-Digital Gene Expression (3’-DGE) Sequencing: High Through-put at Low Cost
June 14, 2022
The RNA-Seq workflow normally begins with the isolation of RNA and continues with library prep. But RNA can be scary to work with! The scourge of lurking RNases has caused countless restless nights for researchers planning their first (or hundredth!) RNA extraction. Read about an alternative method of library prep, 3'-Digital Gene Expression (3'-DGE) which reduces sequencing depth requirements, the cost of sequencing, and has the potential to deliver good differential gene expression results where conventional RNA-Seq might fail.
Read More
RNA Methylation Contributes to mRNA Mobility and Root Growth in Plants
August 6, 2019
RNA mobility in plants, which involves the transport of RNA to different plant tissues, is a phenomenon that has been known to exist for years. However, the mechanism involved, and the biological roles of this activity are not fully understood. RNA methylation might be the answer.
Read More
<< Back to MOTIFvations Blog Home Page